Basic HTML Version

International Journal of Marine Science 2013, Vol.3, No.20, 158-165
http://ijms.sophiapublisher.com
159
al., 2000). Observing the spread of
C. racemosa
in
situations of environmental crisis and rarefaction of
autochthonous populations, authors of early studies
claimed that it was a stress-tolerant species and a
possible indicator of active environmental disturbance
(Buia et al., 1998).
The coastal stretch at Ansedonia (Orbetello, southern
Tuscany, Italy; Figure 1) had a back reef area of
Posidonia oceanica
(L.) Delile with a habitat
characterized by distinctive mixed meadow communities
of macroalgae and seagrass. Since 2003 the mixed
meadow suddenly disappeared (Lenzi et al
.
, 2007),
replaced in 2004-2005 by
C. racemosa,
which spread
shoreward from the barrier reef of
P. oceanica
.
C.
racemosa
biomass showed an increase of two orders
of magnitude between July 2005 and July 2006
(Birardi et al., 2008), confirming the considerable
substrate-covering capacity and rapid development
shown by this species in other parts of the
Mediterranean Sea and also its aggressiveness on
shallow sheltered bottoms with dead
mattes
.
As a result of this sequence of events, we considered it
important to continue monitoring the phytobenthic
settlement dynamics of Santa Liberata back-reef and
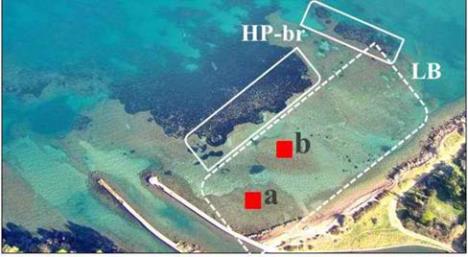
Figure 1 The study area at Santa Liberata (southern Tuscany,
Italy)
Note: HP-br,
Posidonia oceanica
barrier reefs (continuous line);
LB, back reef area (dashed line); squares a and b, plots used for
sampling and estimating plant cover
barrier-reef areas. We therefore monitored cover and
biomass of phytobenthic flora in the back-reef in order
to assess any differences with respect to the previous
spread of
C. racemosa
and to describe the biomass
ratio of this species to other phytobenthic species. Our
aims were: to understand the role of this invader in the
colonisation of degraded areas; to assess whether its
aggressive nature can effectively prevent reconstitution
of the original community; to assess whether it can
also attack and create critical conditions for
P. oceanica
reef areas.
1 Results
Floristic lists with specific cover values for the survey
of August 2011 are reported in Table 1, where they are
compared with July 2005 and 2006 survey results. The
number of species changed from 32 to 30 and 38 in
2005, 2006 and 2011, respectively. The species with
highest cover were:
Jania rubens
v.
rubens
,
Padina
pavonica
,
C. racemosa
,
Cladophora prolifera
,
Penicillus
capitatus
and
Nanozostera noltii
in all three years, and
also
Dictyota dichotoma
and
Cladophora
sp. in 2011.
C. racemosa
showed a decrease in cover in 2011,
returning to 2005 levels, while
N. noltii
increased its
cover in 2011. Phytobenthos still consisted mainly of a
thin compact mat, dominated by the dense texture of
J.
rubens
and
Cladophora
spp., with a prevalence of the
latter, from which emerged sparse
N. noltii
leaves and
tufts of
P. pavonica
and
D. dichotoma
, that sometimes
developed into extensive patches.
The results of correspondence analysis of cover data
of the lists of species in 2005, 2006 and 2011 are
reported in the biplot of Figure 2, where species are
given progressive numerical values in the alphabetical
order of Table 1. We found that: a) the species coded 6,
8, 14, 15, 18, 20, 21, 22, 40, 43, 44, 46 and 39
(superimposed on the graph of Figure 2) were
abundant in 2011 and absent in the other two years; b)
species 34, 25, 11 and 9 were present in 2005 and
2006 and absent in 2011; c) species 19 was only
present in 2005; d) species 17 was present in 2006 and
2011; e) species 1, 2, 5, 10, 12, 16, 24, 26, 28, 30, 31,
32, 33, 36, 37, 38, 41, 42, 45 and 47 (superimposed on
the graph of Figure 2) had no influence on the results
because they were always present. The year 2011
plotted on the positive semiaxis of the abscissa
because it was characterised by abundance of the
species listed in point a), confirming the peculiar nature

